Elanco Announces FDA Approval and Launch of Zenrelia™ (ilunocitinib tablets), Offering An Effective, Safe Solution in Canine Dermatology
-
U.S. Food and Drug Administration approves Zenrelia, a once-daily oral JAK inhibitor for dogs with allergic and atopic dermatitis - Elanco enters the estimated
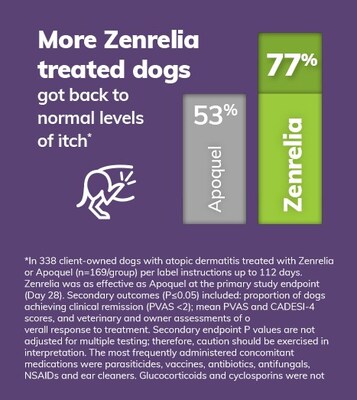
$1.7 billion global canine dermatology market, highly accretive to existing portfolio - In a head-to-head study, Zenrelia was shown to be at least as effective as the market incumbent JAK inhibitor at the primary end point, with an additional endpoint at which Zenrelia got 77% of dogs to clinical remission of itch, compared to 53% of dogs treated with Apoquel® (oclacitinib tablet)1*
- Zenrelia launch begins in
U.S. with Elanco now taking orders; product expected to ship in coming days
Itching is one of the top reasons pet owners bring their dog to the veterinarian, and pet owners and veterinarians want more canine dermatology options. Approximately 17 million dogs suffer from allergic skin disease, including atopic dermatitis, food allergies or flea sensitivity2. Among pet owners who say their dog's itch is not under control, 60% say they've tried treating the itch, but nothing works.3
The approval of Zenrelia represents an important advancement in treating itchy dogs suffering from chronic, acute or seasonal itch and inflammation in a single, once daily tablet from the start. Zenrelia targets itch where it starts by blocking the pathways involved in allergic itch to break the itch-scratch cycle.4 Zenrelia offers visible improvement from the first dose and minimizes the risk of "rebound itch" which affects many dogs treated with the competitive JAK inhibitor.5-10
"Today is a historic day for Elanco with our first of several expected entries into the fast-growing global canine dermatology market, bringing veterinarians and pet owners a highly effective new solution that got more dogs back to normal levels of itch in a head-to-head study with the current JAK inhibitor on the market1*," said
Promising Results from a Head-to-Head Study1
Elanco conducted a head-to-head noninferiority study comparing the efficacy and safety of Zenrelia and Apoquel for submission in the
- Zenrelia provided consistently greater relief from itch and skin lesions over time, with once-daily dosing from the start while rebound itch was observed in Apoquel treated dogs after dosing decreased to once daily after Day 14.
- Zenrelia got more dogs back to normal levels of itch by the end of the study, with 77% of Zenrelia treated dogs achieving clinical remission of itch, compared to 53% of Apoquel treated dogs.
- The difference in treatment response was recognized by owners and veterinarians, with both groups rating Zenrelia higher than Apoquel for overall response to treatment from Day 28 through to Day 112.
- The adverse event profile was similar between the Zenrelia and Apoquel groups.
Veterinarians And Pet Owners Need More Options
Research shows that nearly 70% of veterinarians would be willing to stock another dermatology product2 as there are still too many dogs that aren't getting itch relief and many pet owners that would benefit from a more affordable option.
"We're dedicated to solving these unmet needs in canine dermatology," said
Take for example, Trooper, a one-year-old Yorkshire Terrier who enrolled in the Zenrelia clinical trial. Prior to participating in the clinical trial, Trooper's itch level was 10 out of 10 on the pruritus visual analog scale (PVAS), a validated observation scale for canine itch. By the end of the first two weeks of treatment, he was back to a normal itch level of 1.9 and finished the clinical trial with an itch level of 1.1.** A PVAS score of less than 2 is considered a normal level of itch, also referred to as clinical remission of itch. You can read more about Trooper's story here.
"I was excited to participate as a clinical investigator in the Zenrelia field study because it is clear we need more treatment options for itchy dogs," said Dr.
Demonstrated Safety
The safety of Zenrelia has been demonstrated in multiple toxicity and clinical safety studies. The required margin of safety study for Zenrelia was conducted in healthy dogs dosed with placebo, 1, 2, 3 or 5 times the label dose daily for six months. All dogs completed the study with no serious adverse events.
The Zenrelia label includes a boxed warning on safety related to concurrent vaccine administration based on the results of a vaccine response study. In this study, eight, 10-month-old laboratory beagles received primary vaccinations while being treated with Zenrelia at 3X the label dose. Two dogs were immunosuppressed and euthanized during the study. Antibody responses were evaluated following vaccination. All but one dog responded successfully to modified live vaccines, and two of six dogs responded to inactivated Rabies vaccine at the primary endpoint.
Dogs should be up to date on vaccinations prior to starting Zenrelia. It's important for veterinarians to read the entire package insert, including the Boxed Warning, before prescribing Zenrelia.
"Zenrelia has been demonstrated to be safe and highly effective in a number of studies," said Dr. Mara Tugel, veterinarian and Dermatology Medical Strategic Lead at Elanco. "We recognize that veterinarians need clinically relevant data to guide treatment choices, and plan to pursue additional studies to evaluate vaccine response in Zenrelia-treated dogs. We will continue to work to improve the label over time."
Veterinarians in the
Elanco will conduct a conference call on
ABOUT ELANCO
INDICATIONS
is indicated for control of pruritus associated with allergic dermatitis and control of atopic dermatitis in dogs at least 12 months of age.
IMPORTANT SAFETY INFORMATION
Read the entire package insert before using this drug, including the boxed warning. For Full prescribing information call 1 888 545 5973 or visit www.elancolabels.com/us/zenrelia.
WARNING: VACCINE-INDUCED DISEASE AND INADEQUATE IMMUNE RESPONSE TO VACCINES. Based on results of the vaccine response study, dogs receiving Zenrelia are at risk of fatal vaccine-induced disease and inadequate immune response to vaccines. Discontinue Zenrelia for at least 28 days to 3 months prior to vaccination and withhold Zenrelia for at least 28 days after vaccination. Dogs should be up to date on vaccinations prior to starting Zenrelia. Do not use in dogs less than 12 months old or dogs with a serious infection. Monitor dogs for infections because Zenrelia may increase susceptibility to opportunistic infections. Neoplastic conditions (benign and malignant) were observed during clinical studies. Consider the risks and benefits of treatment in dogs with a history of recurrence of these conditions. The most common adverse reactions were vomiting, diarrhea and lethargy. Zenrelia has not been evaluated in breeding, pregnant, or lactating dogs and concurrent use with glucocorticoids, cyclosporine, or other systemic immunosuppressive agents has not been tested. For full prescribing information see package insert. Forward-Looking Statements: TBD
Zenrelia, Elanco and the diagonal bar logo are trademarks of Elanco or its affiliates. Apoquel is a trademark of
Cautionary Statement Regarding Forward-Looking Statements
This press release contains forward-looking statements within the meaning of the federal securities laws, including, without limitation, statements concerning Zenrelia as a treatment for dogs with allergic dermatitis and timeline for launch, commercial uptake, as well as future studies, publications and other milestones related to Zenrelia, and reflects Elanco's current beliefs and expectations. However, as with any animal health pharmaceutical product, there are substantial risks and uncertainties in the process of drug research, development, and commercialization. Among other things, there is no guarantee that planned or ongoing studies will be completed as planned, that future study results will be consistent with study results to date, that future studies will receive regulatory approval, or that Elanco will execute its strategy as expected. For further discussion of these and other risks and uncertainties that could cause actual results to differ from Elanco's expectations, see Elanco's Form 10-K and Form 10-Q filings with the
Investor Contact:
Media Contact: Season Solorio (765) 316-0233 season.solorio@elancoah.com
References:
-
Elanco Animal Health . Data on File. - AVMA Sourcebook. Elanco, Dermatology Products SOV Study, 2023
-
Elanco and FleishmanHillard TRUE Global Intelligence Survey . -
Elanco Animal Health . Data on File. - Cosgrove SB, et al. A blinded, randomized, placebo-controlled trial of efficacy and safety of the Janus kinase inhibitor oclacitinib (Apoquel®) in client-owned dogs with atopic dermatitis. Vet Dermatol, 2013; 24: 587-e142.
- Little PR, et al. A blinded, randomized clinical trial comparing the efficacy and safety of oclacitinib and ciclosporin for the control of atopic dermatitis in client-owned dogs. Vet Dermatol, 2015; 26: 23-e8.
- Fukuyama T, et al. Demonstration of rebound phenomenon following abrupt withdrawal of the JAK1 inhibitor oclacitinib.
European Journal of Pharmacology , 2017; 794: 20-26. - Takahashi J, et al. Efficacy and safety of 0.0584% hydrocortisone aceponate topical spray and systemic oclacitinib combination therapy in dogs with atopic dermatitis: a randomized, double-blinded, placebo-controlled trial. Vet Dermatol, 2021; 32: 119-e35.
- Denti D, et al. Prolonged twice-daily administration of oclacitinib for the control of canine atopic dermatitis: a retrospective study of 53 client-owned atopic dogs. Vet Dermatol, 2022; 33: 149-e42.
- Olivry T, et al. A randomized controlled trial testing the rebound-preventing benefit of four days of prednisolone during the induction of oclacitinib therapy in dogs with atopic dermatitis. Vet Dermatol, 2023; 34: 99-106.
*Zenrelia was as effective as Apoquel at the primary study endpoint (Day 28). Additional outcomes (P≤0.05) included: proportion of dogs achieving PVAS <2; Mean PVAS and CADESI-4 scores, and veterinary and owner assessments of overall response to treatment. Additional endpoint P values are not adjusted for multiple testing; therefore, caution should be exercised in interpretation.
** Individual results may vary. In the
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/elanco-announces-fda-approval-and-launch-of-zenrelia-ilunocitinib-tablets-offering-an-effective-safe-solution-in-canine-dermatology-302253609.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/elanco-announces-fda-approval-and-launch-of-zenrelia-ilunocitinib-tablets-offering-an-effective-safe-solution-in-canine-dermatology-302253609.html
SOURCE